INTRODUCTION: PNH, a rare, chronic, life-threatening disease, is characterized by hemolytic anemia due to uncontrolled activity of the complement alternative pathway (AP), bone marrow failure, and thrombosis. Inhibition of C5 by intravenously administered eculizumab and ravulizumab reduces intravascular hemolysis, but PNH red blood cells (RBCs) become opsonized and susceptible to extravascular hemolysis (Risitano et al, Blood 2009). Only approximately half of PNH patients become transfusion independent with eculizumab treatment (Hillmen et al, NEJM 2006).
BCX9930 is a potent, selective, orally administered inhibitor of complement factor D. Inhibition of factor D may prevent both intravascular and extravascular hemolysis in PNH. In healthy subjects, BCX9930 showed linear pharmacokinetics and dose-related AP suppression, and was safe and generally well-tolerated over a wide dose range. Here we describe safety and laboratory data establishing proof-of-concept for BCX9930 monotherapy in PNH patients in Study BCX9930-101 (NCT04330534).
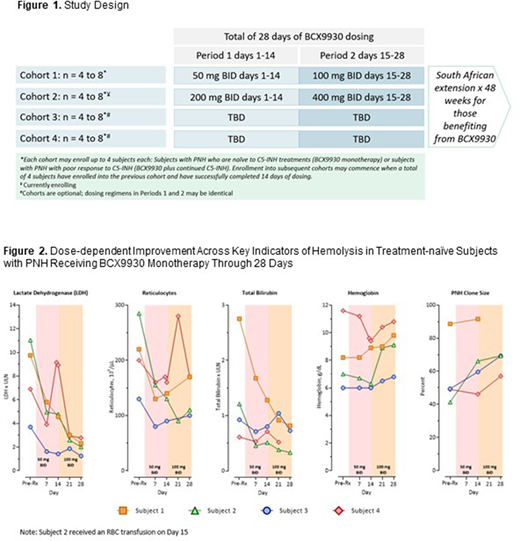
METHODS: Ongoing Study BCX9930-101 includes an open-label, dose-ranging evaluation of BCX9930 in PNH subjects who may either be naïve to C5 inhibitors (and receive BCX9930 as monotherapy) or have an incomplete treatment response to eculizumab or ravulizumab (with BCX9930 added to existing treatment). Up to 4 sequential cohorts each use a forced titration design for the first 28 days (Figure 1). Subjects enrolled in South Africa can participate in an individualized 48-week extension if they derive benefit at Day 28.
Clinical benefit from BCX9930 is evaluated using laboratory monitoring and symptom assessment. Safety and tolerability are evaluated via clinical and laboratory monitoring, causality of adverse events is assessed by investigators, and the study is overseen by an independent Data Monitoring Committee. Data from Cohort 1 through 28 days is reported; data from the extension and subsequent cohorts will be subsequently summarized as available.
RESULTS: To date, four C5 inhibitor naïve PNH subjects in South Africa have enrolled in Cohort 1. These subjects had PNH for a median of 4.5 years; 2 subjects had a history of transfusions in the past year; 1 subject each had a history of aplastic anemia or major thrombosis. Pre-treatment lactate dehydrogenase (LDH), total bilirubin, hemoglobin (Hb), reticulocyte count, and RBC PNH Type III clone size ranged from 3.7-11.1 × ULN, 0.61-3.3 mg/dL, 6.1-11.6 g/dL, 0.13-0.29 × 106/µL, and 41.4%-88.6% respectively.
Treatment over 28 days with 50 mg twice daily (BID; Days 1-14) and 100 mg BID (Days 15-28) of BCX9930 produced dose-dependent, clinically meaningful improvements across hemolysis biomarkers (Figure 2). Decreases were observed in LDH (4/4), reticulocytes (4/4), and total bilirubin (2/2 subjects with elevated pre-treatment values). Increases were observed in Hb (3/4) and PNH RBC clone size (4/4). One subject showed an initial response to BCX9930 50 mg BID, followed by worsening indicators of hemolysis temporally associated with an upper respiratory tract infection (URTI; onset on Day 7). With an increase in dose to 100 mg BID and resolution of the URTI, LDH and reticulocytes fell and Hb rose. All four subjects reported one or more PNH-associated symptoms, including hemoglobinuria, jaundice, fatigue, erectile dysfunction, headache and abdominal pain, prior to enrollment. With the exception of one subject with persistent hemoglobinuria, all symptoms resolved by Day 28 on BCX9930.
Three subjects experienced moderate headache that resolved in < 3 days after initiating BCX9930. One subject developed a rash during treatment with amoxicillin for an URTI; the rash resolved while continuing BCX9930 dosing. One subject on concomitant chronic corticosteroids and azathioprine had an unrelated fatal serious adverse event of disseminated varicella during the study extension. Based on review of safety data, Cohort 2 opened at doses of 200 mg BID and 400 mg BID and, in the 3 subjects who continued into the extension, the dose was titrated to ≥ 200 mg BID.
CONCLUSIONS: Oral BCX9930 elicited rapid changes in laboratory parameters indicative of reduced hemolysis and clinical benefit and was safe and generally well-tolerated over a 28-day dosing interval. These interim results establish proof of concept for monotherapy with BCX9930 in the treatment of C5-inhibitor naïve PNH patients and support evaluation of higher doses.
Kulasekararaj:Alexion:Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants, Speakers Bureau;Ra Pharma:Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants, Speakers Bureau;BioCryst Pharmaceuticals, Inc.:Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees;Apellis:Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants, Speakers Bureau;Roche:Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau;Novartis:Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants, Speakers Bureau;Celgene:Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel grants, Speakers Bureau.Malherbe:Key Oncologics:Honoraria, Other: Conference sponsor;Novartis:Other: Conference sponsor;Astellas:Honoraria, Other: Conference sponsor;Takeda:Consultancy;Acino:Honoraria;Shire:Other: Conference sponsor;BioCryst Pharmaceuticals, Inc.:Consultancy;Janssen:Consultancy, Honoraria, Other: Conference sponsor;Roche:Honoraria, Other: Conference sponsor.McDonald:venetoclax advisory board in South Africa (in CLL context):Consultancy;Alberts Cellular Therapy:Current Employment.Cornpropst:BioCryst Pharmaceuticals, Inc.:Current Employment.Collis:BioCryst Pharmaceuticals, Inc.:Current Employment.Davidson:BioCryst Pharmaceuticals, Inc.:Current Employment.Chen:BioCryst Pharmaceuticals, Inc.:Current Employment.Tower:BioCryst Pharmaceuticals, Inc.:Current Employment.Gesty-Palmer:BioCryst Pharmaceuticals, Inc.:Current equity holder in publicly-traded company, Ended employment in the past 24 months.Sheridan:BioCryst Pharmaceuticals, Inc.:Current Employment.Risitano:Alexion:Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau;Alnylam:Research Funding;Novartis:Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau;Pfizer:Speakers Bureau;Achillion:Membership on an entity's Board of Directors or advisory committees;Apellis:Membership on an entity's Board of Directors or advisory committees, Speakers Bureau;Biocryst:Membership on an entity's Board of Directors or advisory committees;RA pharma:Research Funding;Amyndas:Consultancy;Samsung:Membership on an entity's Board of Directors or advisory committees;Roche:Membership on an entity's Board of Directors or advisory committees;Jazz:Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal